The Medicines and Healthcare products Regulatory Agency (MHRA) and the National Institute for Health and Care Excellence (NICE) have launched the Accelerated Aligned Pathway pilot, a groundbreaking initiative designed to streamline the regulatory and health technology assessment (HTA) processes for innovative medicines in the UK.
First announced in October 2025 and effective from early 2026, this program synchronizes MHRA licensing decisions with NICE value assessments, eliminating the traditional 90-day gap between marketing authorization and NICE guidance.

For small and mid-size biotechs and pharma companies navigating post-Brexit challenges, this pathway offers a faster route to NHS patient access, potentially shortening timelines by 3–6 months while enhancing the UK’s appeal as a competitive hub for global R&D.As of January 2026, the MHRA has reported a 9% rise in clinical trial applications in 2025 compared to 2024, signaling growing confidence in the UK’s reformed landscape.
With additional changes like a 14-day assessment route for Phase 1 trials and refinements to the Innovative Licensing and Access Pathway (ILAP), the UK is positioning itself for accelerated innovation.
Background and Objectives of the Accelerated Aligned Pathway
The pathway builds on the MHRA’s 2025 Regulatory Action Plan and responds to stakeholder calls for reduced administrative burdens in a post-Brexit era. Traditionally, MHRA approvals precede NICE appraisals, creating delays that impact revenue and patient access. The pilot addresses this by enabling parallel workflows under a joint information-sharing agreement between MHRA and NICE.
Key objectives include:
- Reducing timelines: Simultaneous publication of licensing and guidance decisions.
- Enhancing efficiency: Cutting business costs by 25% through smarter regulation.
- Prioritizing innovation: Focusing on therapies for unmet needs, such as oncology, rare diseases, and advanced modalities (e.g., gene/cell therapies).
- Gathering feedback: Early adopters help refine the full rollout, including a new joint scientific advice service by April 2026.
This aligns with broader MHRA reforms, including ILAP refinements (updated January 21, 2026) for transformative products and a stepwise approach to Phase 1 assessments.
Eligibility Criteria
The pilot is open to companies with innovative medicines designated for “early access” under MHRA criteria—those addressing significant unmet needs with promising benefit-risk profiles. Eligibility includes:
- Products in late-stage development or nearing submission.
- Alignment with NHS priorities (e.g., rare diseases, health inequalities).
- Drug-device combinations if they fit ILAP standards.
- Non-commercial developers, broadening access for SMEs and academia.
Exclusions apply to routine improvements or non-novel products. Sponsors with scheduled NICE appraisals should email [email protected] for a suitability check.
Process and Support Mechanisms
- Early Registration: Contact NICE to assess eligibility, providing details on innovation status.
- Parallel Submissions: Use MHRA’s eCTD for licensing and NICE templates for HTA, with harmonized data requirements.
- Joint Review: Synchronized assessments culminate in simultaneous decisions.
- Scientific Advice: A single-entry service (launching April 2026) offers coordinated pre-market guidance on evidence, trial design, real-world data (RWD), and economic modeling.
- NHS Integration: Includes priority scheduling and adoption planning to address uptake barriers.
The pilot runs through 2026, with quarterly feedback loops and a mid-2026 evaluation.
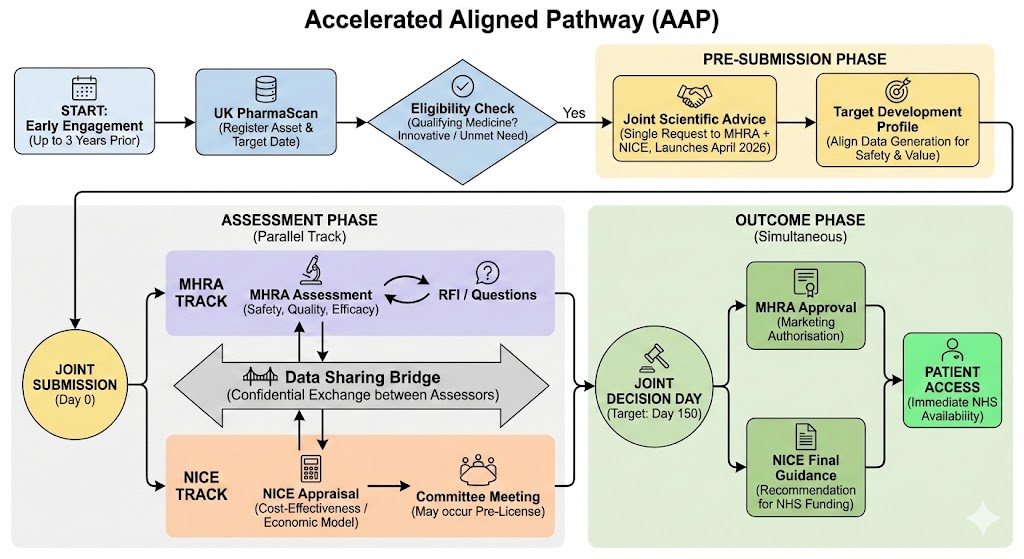
Timelines
- Standard MHRA: 150 days for new active substances.
- Pilot Benefit: Minus the 90-day NICE lag; overall 3–6 months faster to patient access.
- Phase 1 Fast-Track: 14-day assessment (new in 2026).
- ILAP Tie-In: Rolling reviews for eligible products.
Implications for Small and Mid-Size Biotechs and Pharma Companies
For SMEs, the pathway reduces red tape and capital strain, enabling quicker ROI on R&D. It enhances predictability—early joint advice minimizes resubmissions—and positions the UK as a “first-launch” market alongside the EU. However, success requires robust dossiers aligned for dual regulatory/HTA scrutiny. With MHRA’s 9% trial application surge in 2025, early movers can leverage this momentum for multinational strategies.

How Pharma Design Limited Can Help
At Pharma Design Limited, we specialize in bridging EU-UK regulatory gaps for small and mid-size biotechs and pharma companies. Our tailored services ensure you’re pilot-ready:
- Regulatory Strategy Audits: Assess your pipeline for eligibility; align EU EMA and UK MHRA dossiers to avoid divergence.
- ILAP and Pathway Preparation: Guide Innovation Passport applications and joint scientific advice requests, including RWD strategies via CPRD/Genomics England.
- Orphan and Unmet Needs Expertise: Support UK Orphan Designation and rare disease filings, drawing on our track record in ATMPs and oncology.
- Market Access Consulting: Navigate parallel NICE reviews, including economic modeling and managed access agreements.
- Paediatric Support: Ensure compliance with PIP well in advance of your MAA and verify post-marketing obligations.
We’ve helped clients cut UK MAA timelines by months through proactive alignment. If you’re an SME eyeing the UK market, contact us for a free pipeline review—let’s accelerate your path to patients.
