In the competitive landscape of pharmaceutical development, regulatory affairs play a pivotal role in bringing innovative therapies to market. For developers, particularly those based outside Europe, navigating the complexities of EU and UK regulations can be resource-intensive.
At Pharma Design Limited, a UK-based regulatory boutique consultancy, we provide targeted expertise to streamline this process. Our services are designed to deliver value through cost efficiency, flexibility, and deep knowledge, as demonstrated through our work with US-based clients. This article outlines how our approach compares favourably to standard Contract Research Organisations (CROs) in terms of cost and operational advantages, based on real-world engagements.
Comprehensive Regulatory Services Tailored for Efficiency
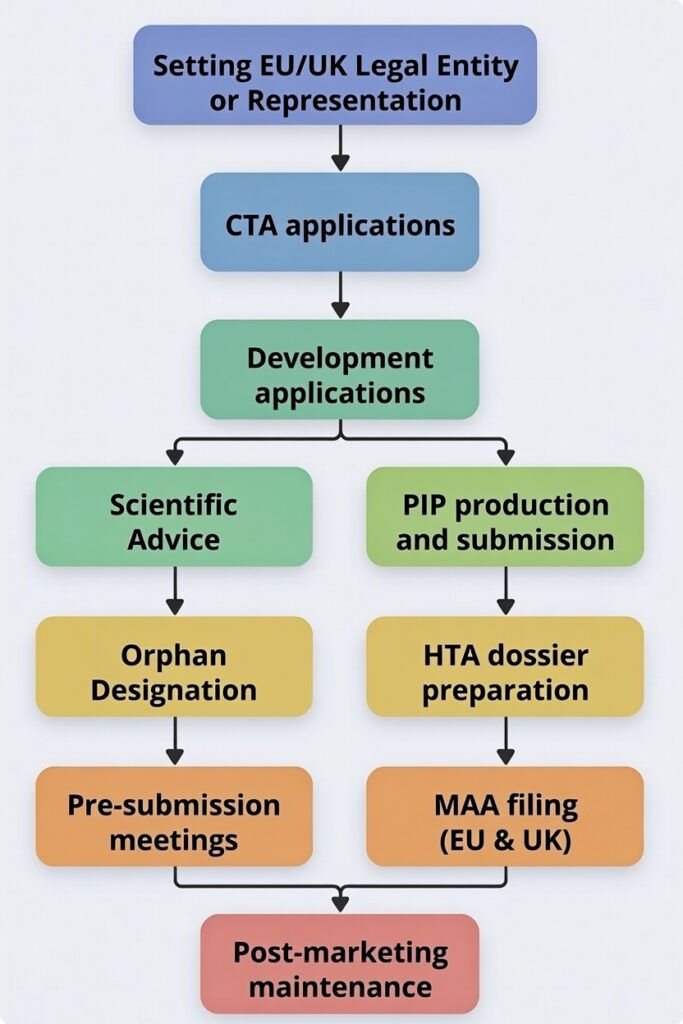
Pharma Design Limited offers end-to-end support for regulatory activities in Europe, focusing on strategic and operational needs from early development through post-marketing. Our core offerings include:
- Clinical Trial Application (CTA) Submissions: Handling multi-country initial submissions in the EU and UK, from preparation to approval.
- Health Authority Interactions: Facilitating communications with agencies like the EMA and MHRA, including pre-submission meetings.
- Company Representation and Setup: Acting as the EU/UK representative and assisting with establishing UK limited companies to access fee waivers or reductions.
- Paediatric Investigation Plans (PIPs): Production and submission to ensure compliance with paediatric requirements.
- Orphan Designation Applications: Preparing and submitting applications to secure orphan status for rare disease therapies.
- Accelerated Pathways: Supporting applications for ILAP (Innovative Licensing and Access Pathway) in the UK and PRIME (PRIority MEdicines) in the EU.
- Health Technology Assessment (HTA) Dossiers: Developing comprehensive dossiers for reimbursement evaluations.
- Marketing Authorisation Application (MAA) Preparation and Submission: Guiding full dossier assembly and filing.
- Post-Marketing Maintenance: Managing variations and ongoing compliance.
These services are delivered by a lean team of seasoned experts, enabling us to maintain lower overheads while providing high-quality output. Unlike larger CROs, which often involve layered structures and standardised processes, our model emphasises direct involvement and adaptability.
Cost Advantages: Delivering More for Less
One of the most tangible benefits of partnering with Pharma Design Limited is our competitive pricing structure, which is significantly lower than that of standard CROs. For instance, our fees for an EU multi-country initial CTA submission range from USD 85,000 to 150,000, covering the full process from submission to approval. This is based on actual project scopes with US clients and reflects our efficient, focused approach.
In comparison, standard CROs typically charge higher rates for similar services due to broader operational scales and additional markups. Industry benchmarks indicate that CRO costs for multi-country CTA submissions in Europe can exceed USD 200,000 to 300,000 or more, depending on complexity and country count. Our lower pricing stems from a streamlined team without unnecessary bureaucracy, allowing us to pass savings directly to clients while maintaining rigorous standards.
This cost-effectiveness extends across our portfolio. For example, orphan designation applications or PIP submissions are priced to reflect the targeted effort required, often 30-50% below CRO equivalents, without compromising on success rates. Our US clients have consistently reported these savings as a key factor in their decision to engage us, enabling them to allocate resources toward core R&D activities.
Flexibility and Responsiveness: Adapting to Real-World Challenges
Pharmaceutical development is rarely linear, with frequent pivots due to emerging data, market shifts, or regulatory feedback. Pharma Design Limited’s lean structure allows us to adapt swiftly—whether changing CTA target countries or sites, repositioning submissions, or modifying amendment packages mid-process. This flexibility is embedded in our operations: we provide immediate feedback through dedicated one-on-one client support, ensuring queries are addressed within hours rather than days.
In contrast, standard CROs often operate with fixed protocols and larger teams, which can lead to delays and additional fees for changes. Our clients appreciate this agility; for instance, in recent projects, we seamlessly adjusted strategies for ILAP/PRIME applications in response to evolving clinical data, avoiding costly rework.
Expertise in Complex Interdependencies
Our team’s extensive experience enables us to interpret regulatory interdependencies and provide holistic advice on complex tasks. This includes navigating convoluted matrices involving CTA, PIP, orphan designations, and HTA preparations simultaneously. By anticipating potential overlaps or conflicts—such as aligning paediatric requirements with orphan benefits—we help clients avoid pitfalls that could inflate costs or timelines.
This proactive guidance has proven effective for US-based developers unfamiliar with EU/UK nuances, resulting in smoother interactions with health authorities and higher approval rates.
Proven Value for Developers
Through our work with US clients, Pharma Design Limited has demonstrated that cost-effective regulatory support does not mean cutting corners. Instead, it means delivering precise, adaptable expertise that aligns with client needs. By choosing us over traditional CROs, developers gain access to lower costs, faster adaptations, and dedicated support—all while advancing their therapies toward market.
If you’re a pharmaceutical developer seeking efficient European regulatory pathways, contact us at Pharma Design Limited to discuss how we can support your program.
Visit our website for more insights or email [email protected].
Pharma Design Limited is committed to factual, non-promotional content. All cost comparisons are based on industry benchmarks and client experiences as of December 2025.
