How to license a medicine for sale in the UK, including national and international routes, and information on fees.
”There are several routes to obtain a marketing authorisation in the United Kingdom (UK), Great Britain (England, Scotland and Wales) or Northern Ireland. The options available will be determined by the intended market and the type of application.”
1. First of all, the Legal Basis of your application needs to be established to identify legislative and regulatory procedures that may apply. The appropriate legal basis will depend the type of application you are making. The options on legal bases are:
-
full application – Regulation 50 (previously Article 8(3) of Directive 2001/83/EC)
-
generic application – Regulation 51
-
hybrid application – Regulation 52
-
similar biological application – Regulation 53
-
well-established use application – Regulation 54
-
fixed-combination application – Regulation 55
-
informed consent application – Regulation 56
-
traditional herbal registrations – Regulation 127
-
certificate of homeopathic medicinal products – (called Simplified Registration scheme)
-
Regulation 103 national homeopathic products (called the National Rules Scheme)
-
Regulation 50(6)(g) and Schedule 10
-
2. Application process
All UK marketing authorisation applications should be submitted through the MHRA Submissions Portal. The application should be submitted using the electronic Common Technical Document (eCTD). The pre-submission checklist (PDF, 129 KB, 7 pages) and the eAF and cover letter tool should be used to determine what documents and information to include in the application. If the correct documents and information are not submitted the application will not be validated. The MHRA will check that eCTD submissions are technically valid using the Lorenz Docubridge validation tool which strictly aligns validation against the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) international standards and eCTD 3.2 regional requirements. It is recommended that a validation tool is used to check the submission. Some of the critical components of an application include:
PL number
If applying for a UK or Northern-Ireland, licence, then a PL number must be sought from the MHRA Portal or by emailing [email protected] before you submit the application.
Summary of product characteristics (SmPC)
The summary of product characteristics (SmPC) should be submitted to the MHRA in the correct format using the SPC template (MS Word Document, 36 KB). If this template is not used the submission will be rejected. The templates should not be altered in any way, other than inserting the relevant information.
Purchase Orders (POs)
It is the applicant’s responsibility to make sure the invoices for submissions are paid on time. If an organisation operates a PO system, please make sure that the relevant PO is provided to the MHRA before the invoice is issued.
Refusal to pay outstanding fees on the grounds that the PO is not provided on the invoice will not be accepted. The use of POs is an internal control process and cannot be used as a reason to withhold payment of legitimate invoices.
Payments
Once an application has been validated, applicants will receive an invoice so that a payment for the outstanding amount can be made. All invoices must be settled upon receipt. Penalty fees may be incurred for non-payment. Details of the penalties are explained in our Fees Regulations.
Non-payment may also result in suspension of any licence or authorisation, followed by legal proceedings for any unpaid amounts, as a debt due to the Crown.
3. National Routes
The rolling review is a route for marketing authorisation applications (MAA) where an applicant for a marketing authorisation submits modules of the eCTD dossier incrementally for pre-assessment by the MHRA rather than as part of a consolidated full dossier submission.
The rolling review is intended to streamline the development of novel medicines by offering periodic enhanced regulatory interaction and advice to reduce the risk of failure at the final phase and may be integrated with the target development profile (TDP) to provide a clearer pathway for development of innovative medicines.
Rolling review can be used for a “full dossier” new active substance (NAS) in the UK or for a similar biological medicinal product (a bio-similar). Supporting data requirements for a new active substance are unchanged from those set out in regulation 50(5) of the Human Medicines Regulations.
The process is a modular approach to submission and evaluation. The quality, non-clinical and clinical data may be submitted separately or jointly, depending on individual circumstances and/or data availability, in the format of common technical modules CTD modules 3-5.
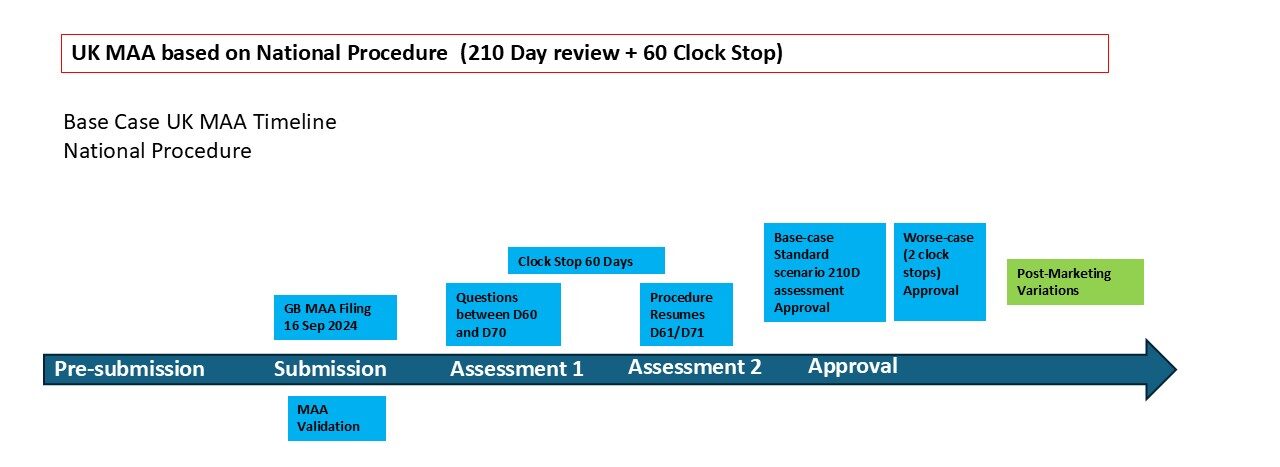
4. MHRA’s national assessment procedure for marketing authorisation applications.
The national assessment procedure guidance applies to national MA applications for both innovative and established medicines, but the requirements, procedures and timetables differ. Timetables are measured in calendar days excluding regulatory clock-stops.
Innovative medicines applications are applicable to the following:
- New active substances (NAS), defined as active substances that have not been authorised in a medicinal product in the UK before
- All biological products (derived or manufactured from a living biological system) including advanced therapy medicinal products (ATMPs), vaccines and bio-similars
- New combinations of existing active substances
- Orphan medicinal products
- Conditional MA or MA under exceptional circumstances
- Line extensions of any of the above (for example, addition of strengths, pharmaceutical forms or routes of administration)
The innovative medicines timetable allows for a positive decision within 150 clock-on days if all issues are resolved following one round of questions. Where there are outstanding issues at Day 150, MHRA will come to a final decision within 210 clock-on days.
Established medicines applications are covered in section 3. These are defined as applications that do not meet the innovative medicines application criteria outlined above. We will come to a final decision on established medicines applications within 210 days. If we have raised only minor issues, there is the opportunity for an earlier decision.
Ask for help from Pharma-Design Limited for your UK filing application. We have successfully assisted clients for may years with different types of applications.